Our Pipeline

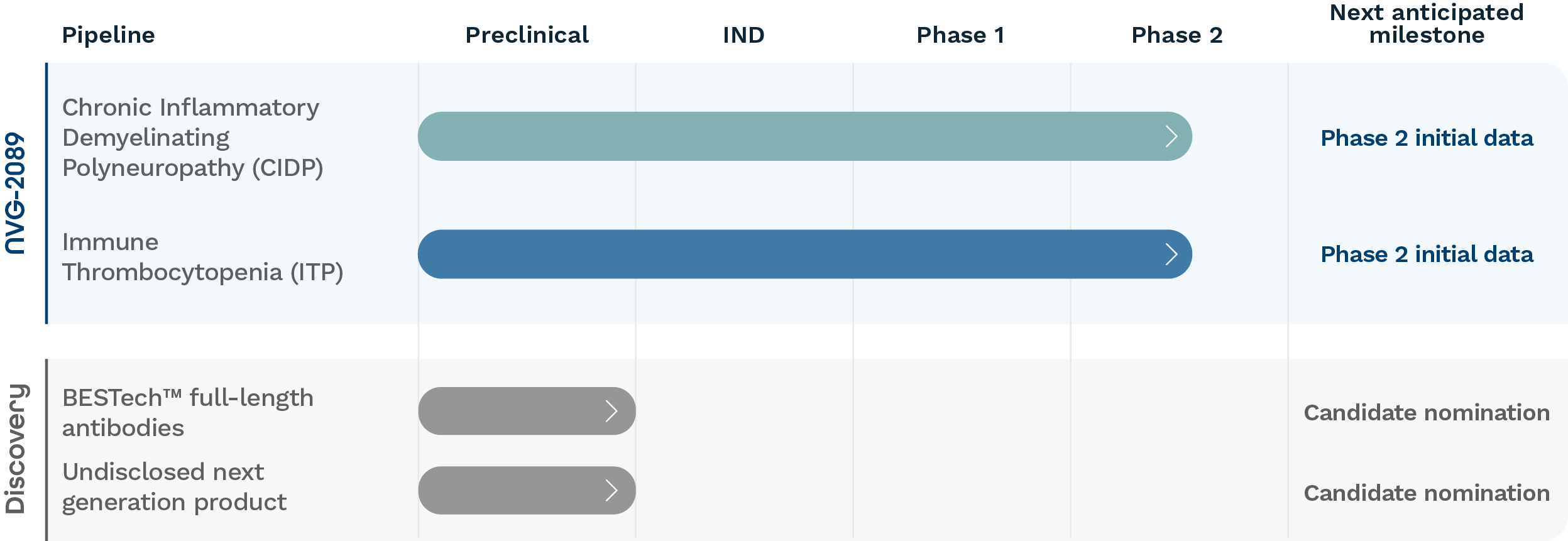
Our Therapies
We aim to bring safe, efficacious immune-modulating therapies to patients with autoimmune disease. We are developing NVG-2089 to treat patients with unmet and severe neurologic and dermatologic autoimmune disease. Our next-generation BESTech™ platform promises to enhance the function of multiple clinically validated drug targets, improving existing treatment across a wide range of autoimmune diseases.
What is CIDP?
Chronic Inflammatory Demyelinating Polyneuropathy is an autoinflammatory neurologic disease characterized by myelin sheath destruction of the peripheral nerves. CIDP leads to motor weakness, sensory impairment, and reduced or absent reflexes in the arms and legs and can present either in a progressive or relapse-remitting but chronic course.
Prevalence
CIDP prevalence in the US is estimated to be 30,000. Among these patients, 60% of are characterized as “typical” CIDP with symmetric sensorimotor polyneuropathy, and 40% are further characterized into either focal, motor, sensory subtypes (~30%) or multifocal, distal subtypes (~10%).
Disease Burden
CIDP patients have a significant disease burden, as symptoms include tingling, weakness, and/or numbness in the extremities, loss of reflexes, and loss of balance. Longitudinal studies have implicated CIDP with a ~14% risk of ambulation loss and ~3% risk of fatality over 18 years. In addition, CIDP is commonly misdiagnosed due to lack of clear biomarkers and similarities with other neuropathy diseases.
Current Treatments
Immunoglobulin (IVIg or SCIg) are the only approved treatments for CIDP patients, and long-term chronic administration for maintenance is required for most patients.
NVG-2089 in Clinical Development
Nuvig’s lead investigational candidate NVG-2089 was previously studied in a Phase 1 study in healthy volunteers, in which NVG-2089 was well tolerated with no serious or severe adverse events reported. NVG-2089 is currently in a Phase 2 clinical trial in Europe and the U.S. (INVGOR) to evaluate the safety, tolerability, and potential clinical benefit in individuals with chronic inflammatory demyelinating polyneuropathy (CIDP) and is also in a Phase 2 clinical trial in Europe and the U.S. (INVOKE) to evaluate the safety, tolerability, and potential clinical benefit in individuals for immune thrombocytopenia (ITP). For more information, contact clinicaltrials@nuvigtx.com.
What is ITP?
Immune thrombocytopenia is a rare autoinflammatory condition characterized by low platelet counts due to immune-mediated platelet destruction and impaired platelet production.
Prevalence
ITP diagnosed prevalence in the US is estimated to be 71,000. Most of these cases (~95%) are characterized as chronic ITP, and 85% of those cases are primary ITP (not caused by an underlying infectious or immune condition).
Disease burden
ITP patients can suffer from bruising, mucosal bleeding, and, in severe cases, life-threatening hemorrhages. Patients also experience chronic fatigue which causes a substantial quality of life burden.
Current treatments
Patients typically start on steroids (+/- IVIg for severe patients who require a rapid response) and then are given oral thrombopoietin receptor agonists or rituximab as maintenance treatment.
NVG-2089 in Clinical Development
Nuvig’s lead investigational candidate NVG-2089 was previously studied in a Phase 1 study in healthy volunteers, in which NVG-2089 was well tolerated with no serious or severe adverse events reported. NVG-2089 is currently in a Phase 2 clinical trial in Europe and the U.S. (INVGOR) to evaluate the safety, tolerability, and potential clinical benefit in individuals with chronic inflammatory demyelinating polyneuropathy (CIDP) and is also in a Phase 2 clinical trial in Europe and the U.S. (INVOKE) to evaluate the safety, tolerability, and potential clinical benefit in individuals for immune thrombocytopenia (ITP). For more information, contact clinicaltrials@nuvigtx.com.
Learn more about our novel approach to rebalancing immune function.